
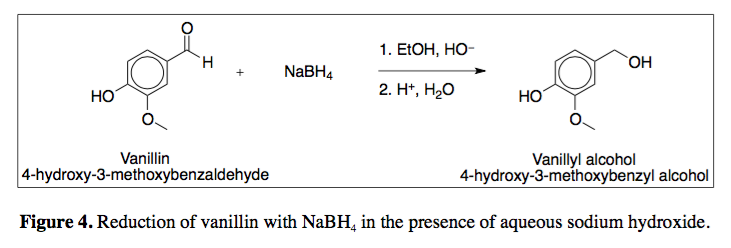
The reaction is carried out in solution in water to which some sodium hydroxide has been added to make it alkaline.
Two possible variants (there are several others!) are:

There are several quite different ways of carrying out this reaction. Sodium tetrahydridoborate (previously known as sodium borohydride) has the formula NaBH 4, and contains the BH 4 - ion. The reduction of aldehydes and ketones by sodium tetrahydridoborate Because of that simplification, these reactions are dealt with entirely on this page - without the "talk through" page that you will find for other mechanisms on this site. Only one UK A level Exam Board (AQA) is likely to ask for these mechanisms, and they are happy with a simplified version of what is quite a complex mechanism. This page gives you the facts and mechanisms for the reduction of carbonyl compounds (specifically aldehydes and ketones) using sodium tetrahydridoborate (sodium borohydride) as the reducing agent. Reduction of carbonyl compounds using sodium tetrahydridoborate


 0 kommentar(er)
0 kommentar(er)
